About
An Optimal Regulation of Fluxes Dictates Microbial Growth In and Out of Steady-State
Griffin Chure and Jonas Cremer
Abstract
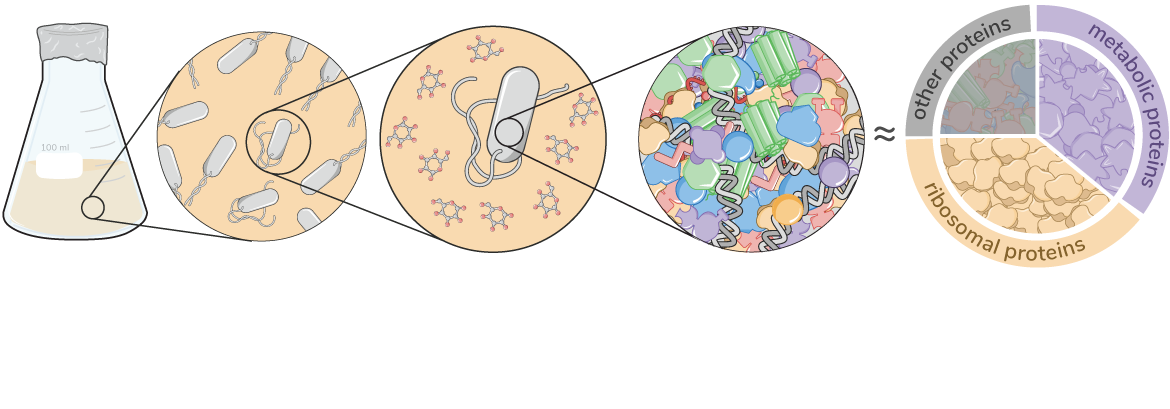
Effective coordination of cellular processes is critical to ensure the competitive growth of microbial organisms. Pivotal to this coordination is the appropriate partitioning of cellular resources between protein synthesis via translation and the metabolism needed to sustain it. Here, we present a coarse-grained and organism-agnostic theory of microbial growth centered on the dynamic regulation of this resource partitioning. At the core of this regulation is an optimization of metabolic and translational fluxes, mechanistically achieved via the perception of charged- and uncharged-tRNA turnover. An extensive comparison with ≈ 50 data sets from Escherichia coli establishes the theory’s veracity and shows that it can predict a remarkably wide range of growth phenomena in and out of steady-state with quantitative accuracy. This predictive power, achieved with only a few biological parameters, cements the preeminent importance of protein synthesis in microbial growth and establishes the theory as an ideal physiological framework to interrogate the dynamics of growth, competition, and adaptation in complex and ever-changing environments.